DNA and the Genetic!
From the cosmic vastness we explored in universe creation to the quantum intricacies of subatomic particles, we now turn our attention to perhaps the most profound mystery of all: the molecular blueprint that defines every living thing on Earth. DNA—deoxyribonucleic acid—represents the ultimate convergence of chemistry, biology, and information science, carrying within its elegant double helix the instructions for all of life’s complexity.pmc.ncbi.nlm.nih+2
The story of DNA is not merely one of scientific discovery, but a testament to human ingenuity in deciphering nature’s most sophisticated code. Today, as we stand on the threshold of a genetic revolution powered by technologies like CRISPR and personalized medicine, understanding DNA has never been more crucial to our future.crisprtx+2
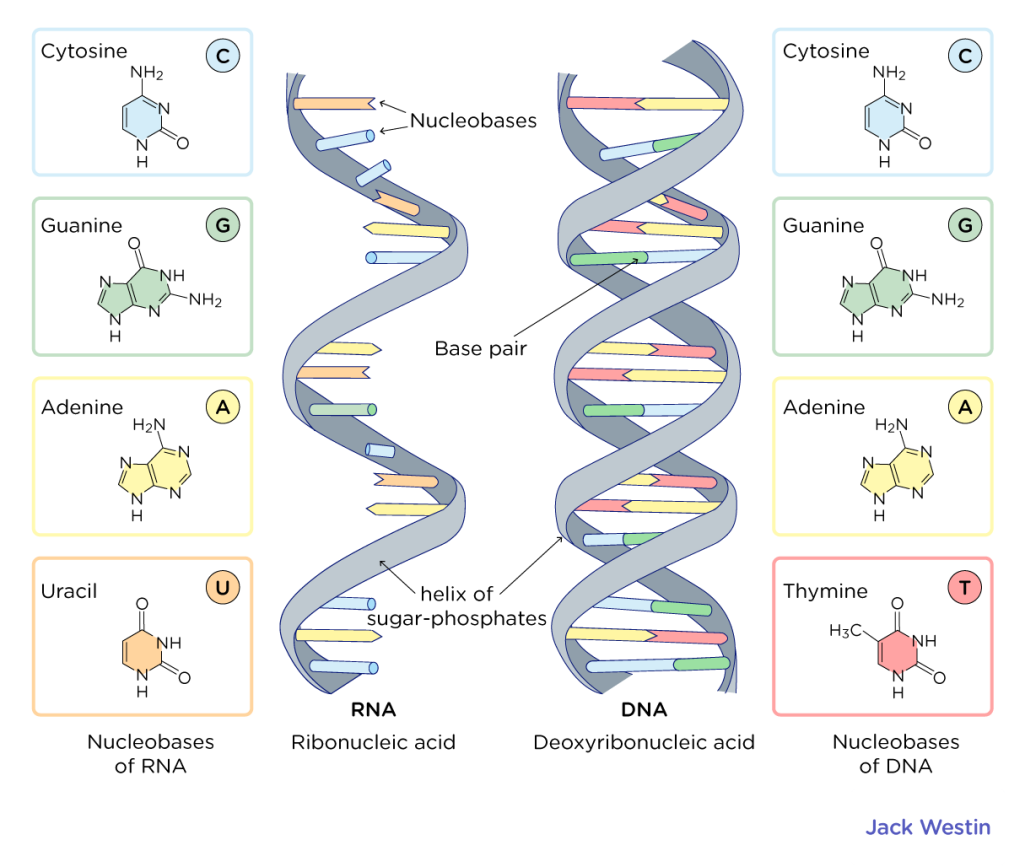
Comparative illustration of RNA and DNA structures showing nucleobases, base pairing, and the sugar-phosphate helix in the DNA double helix Watson-Crick model jackwestin
The Race to Decode Life’s Structure
The discovery of DNA’s double helix structure in 1953 represents one of the most celebrated achievements in scientific history, yet the path to this breakthrough was built upon decades of meticulous research by numerous scientists. Friedrich Miescher first isolated “nuclein” (DNA) from white blood cell nuclei in bandages from a local hospital in 1869, but the significance of his discovery wouldn’t be recognized for generations.yourgenome+3
The true race for DNA’s structure began in earnest after Oswald Avery’s 1944 demonstration that DNA, not protein, was the “transforming principle” responsible for heredity. By the early 1950s, several research teams were converging on the solution, each bringing critical pieces to the puzzle.kci+2
Rosalind Franklin and Maurice Wilkins at King’s College London used X-ray crystallography to probe DNA’s physical structure. Franklin’s meticulous work produced the famous “Photograph 51“—an X-ray diffraction image that clearly revealed DNA’s helical structure and provided crucial measurements of its dimensions. Her data indicated that the phosphate groups were on the outside of the molecule, not inside as some models suggested.journals.iucr+2
Meanwhile, James Watson and Francis Crick at Cambridge University were building physical models, using metal scraps and paper cutouts to test various structural possibilities. When Watson saw Franklin’s Photograph 51, he immediately recognized the telltale diffraction pattern of a helix. Combined with Erwin Chargaff’s rules showing that adenine always equaled thymine and guanine always equaled cytosine in any DNA sample, the pieces finally fell into place.genome+3
On April 25, 1953, Watson and Crick published their landmark paper in Nature, proposing the double helix model with antiparallel strands held together by complementary base pairs. Their model immediately suggested how DNA could replicate: each strand serves as a template for creating its complement, ensuring faithful copying of genetic information across generations.profiles.nlm.nih+2
The Genetic Code: From DNA to Life
The double helix discovery left one fundamental question unanswered: How does DNA’s sequence of just four bases encode the complexity of living organisms? The solution lay in understanding how genetic information flows from DNA to proteins—the molecular machines that carry out life’s functions.yourgenome+2

Diagram of RNA translation illustrating the interaction of mRNA, tRNAs, and ribosome during protein synthesis wikipedia
The genetic code represents one of biology’s most elegant solutions to an information storage problem. With only four DNA bases (A, T, G, C) but twenty different amino acids comprising proteins, the code needed to be more sophisticated than a simple one-to-one correspondence. The answer proved to be a triplet code—each sequence of three consecutive bases (a “codon”) specifies one amino acid.chem.libretexts+2
This coding system is remarkably redundant and robust. With 64 possible three-base combinations (4³) but only 20 amino acids, most amino acids are encoded by multiple codons, providing protection against mutations. Three codons serve as “stop” signals, terminating protein synthesis, while one codon (AUG) serves as both the start signal and codes for methionine.wikipedia+2
Marshall Nirenberg, Har Gobind Khorana, and Robert Holley cracked this genetic code in the 1960s through ingenious experiments. They created artificial RNA sequences with known compositions and observed which amino acids were incorporated into proteins, systematically deciphering each codon’s meaning. Their work revealed that the genetic code is virtually universal across all life forms—from bacteria to humans—underscoring our common evolutionary origin.yourgenome+2
The Human Genome Project: Biology’s Manhattan Project
If discovering DNA’s structure was biology’s equivalent of splitting the atom, then the Human Genome Project was its Manhattan Project. Launched in 1990 with the ambitious goal of sequencing all 3.1 billion base pairs of human DNA, it represented the largest biological undertaking in history.pmc.ncbi.nlm.nih+2
The project faced enormous technical challenges. Early DNA sequencing methods were laborious and expensive, making the prospect of sequencing an entire human genome seem almost impossible. Yet through international collaboration, technological innovation, and unprecedented funding, researchers gradually overcame each obstacle.wikipedia+2
The breakthrough came through a hierarchical shotgun approach. Researchers broke the genome into manageable 150,000 base pair segments, cloned them into bacterial artificial chromosomes, then sequenced each piece separately before assembling the complete picture. Multiple sequencing centers across the United States, United Kingdom, Japan, France, Germany, and China worked in coordinated parallel, sharing data openly.genome+1
Key discoveries from the completed genome shocked the scientific community:wellcome+1
- Humans possess only about 22,300 protein-coding genes—roughly the same as a roundworm and far fewer than initially predictedwikipedia
- Less than 2% of the genome actually codes for proteins; the rest was initially dismissed as “junk DNA” but is now known to have regulatory functionswikipedia
- Humans share 99.9% of their DNA sequence, highlighting our fundamental genetic unitywikipedia
The project’s completion in 2003 marked the beginning of the genomic era. Sequencing costs plummeted from $95 million per genome in 2001 to just $525 in 2022, making personalized genomics accessible to millions. This democratization of genetic information has transformed medicine, agriculture, and our understanding of human evolution.icr+2
CRISPR: The Genetic Revolution
While the Human Genome Project gave us the ability to read life’s instruction manual, CRISPR-Cas9 technology provided the tools to edit it. Discovered in bacterial immune systems and adapted for genome editing by Jennifer Doudna and Emmanuelle Charpentier, CRISPR represents perhaps the most significant biotechnology breakthrough since the discovery of DNA itself.crisprtx+2

Diagram illustrating the stepwise mechanism of CRISPR/Cas9 gene editing from complex assembly to donor DNA insertion ncbi.nlm.nih
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) evolved as bacteria’s adaptive immune system. When viruses attack bacteria, the CRISPR system captures pieces of viral DNA and stores them as molecular “mugshots”. If the same virus attacks again, the bacteria use these stored sequences to guide the Cas9 nuclease to cut the invader’s DNA.wikipedia+1
The genius of CRISPR lies in its programmability. Scientists can design guide RNAs that direct Cas9 to virtually any DNA sequence, allowing precise cuts at desired locations. Once cut, cells’ natural repair mechanisms can be harnessed to delete, insert, or modify genes.crisprtx+2
CRISPR applications span from basic research to clinical medicine:pmc.ncbi.nlm.nih+1
- Gene therapy: Correcting disease-causing mutations in patients with inherited disorderswikipedia+1
- Cancer treatment: Engineering immune cells to better recognize and attack tumorsnature+1
- Agricultural improvement: Creating crops with enhanced nutrition, disease resistance, or climate adaptationsapac.illumina+1
- Basic research: Creating cellular and animal models of human diseasespmc.ncbi.nlm.nih
Recent breakthroughs demonstrate CRISPR’s growing sophistication. Base editors can make single-letter changes to DNA without cutting both strands. Prime editing allows insertion of specific sequences with unprecedented precision. Epigenetic editing can modify gene expression without changing DNA sequence.news.stanford+1
Personalized Medicine: Healthcare’s Genetic Future
The convergence of genomic knowledge and advanced analytics is ushering in an era of personalized medicine—healthcare tailored to individual genetic profiles. Rather than the traditional “one size fits all” approach, doctors can now select medications and treatments based on a patient’s genetic makeup.pmc.ncbi.nlm.nih+3

A circular diagram showing the integration of genomic and clinical data with variant interpretation, genetic counseling, patient information, and EMR access in personalized medicine nature
Pharmacogenomics—the study of how genes affect drug response—exemplifies personalized medicine’s potential. Genetic variations can dramatically alter how patients metabolize medications, leading to treatment failures or dangerous side effects. By testing for these variants, physicians can prescribe the right drug at the right dose from the start.pmc.ncbi.nlm.nih+3
Cancer treatment has been revolutionized by genomic insights. Rather than classifying cancers solely by their organ of origin, oncologists increasingly categorize them by their genetic alterations. Drugs originally developed for one cancer type prove effective against others driven by the same mutations. Targeted therapies attack specific molecular pathways disrupted in cancer cells while sparing healthy tissue.icr+1
The GIFT Model (Genomic, Imaging, Function, and Therapy) represents a comprehensive framework for implementing personalized medicine. This approach integrates:pmc.ncbi.nlm.nih
- Genomic profiling to identify disease-causing variants
- Advanced imaging to visualize disease progression
- Functional analysis to understand how genetic changes affect cellular processes
- Targeted therapy based on molecular insightspmc.ncbi.nlm.nih
Epigenetics: Beyond the Genetic Code
While DNA sequence provides life’s blueprint, epigenetics—literally “above genetics”—determines how that blueprint is interpreted. Epigenetic modifications don’t change DNA sequence but alter gene expression patterns, adding layers of regulatory complexity.clinicalepigeneticsjournal.biomedcentral+4
DNA methylation, the most studied epigenetic modification, typically silences genes when methyl groups are added to cytosine bases. Histone modifications—chemical changes to the proteins around which DNA is wrapped—can either activate or repress nearby genes. Non-coding RNAs provide another layer of regulation, fine-tuning gene expression in response to cellular conditions.begellhouse+5
Epigenetic modifications are environmentally responsive and potentially heritable. Diet, stress, toxin exposure, and other environmental factors can alter epigenetic marks, potentially affecting not only an individual but their offspring. This provides a mechanism for rapid adaptation to environmental changes without waiting for genetic mutations.pmc.ncbi.nlm.nih+2
Understanding epigenetics has profound implications for medicine. Many diseases, from cancer to diabetes, involve epigenetic disruptions. Unlike genetic mutations, epigenetic changes are potentially reversible, making them attractive therapeutic targets. Epigenetic drugs already approved for cancer treatment work by restoring normal gene expression patterns.nature+3
Synthetic Biology: Engineering Life
As our understanding of genetics deepens, scientists are moving beyond simply reading and editing natural genetic systems to engineering entirely new ones. Synthetic biology applies engineering principles to biology, designing organisms with novel functions not found in nature.sapac.illumina+2
Synthetic biologists treat biological systems as programmable machines. They design genetic circuits that function like electronic circuits, with inputs, outputs, and logical operations. Engineered bacteria can be programmed to detect environmental toxins, produce pharmaceuticals, or even perform computational operations.nature+3
Applications span multiple industries:lifesciences.danaher+1
- Biomanufacturing: Engineering microorganisms to produce complex molecules like pharmaceuticals or biofuelssapac.illumina+1
- Medicine: Creating living therapeutics that can respond dynamically to disease conditionswikipedia+1
- Environmental remediation: Designing organisms to clean up pollution or capture carbon dioxidepmc.ncbi.nlm.nih+1
- Agriculture: Developing crops with entirely new capabilities, from enhanced nutrition to environmental sensingpmc.ncbi.nlm.nih+1
The field faces significant challenges in moving from laboratory to real-world applications. Biological systems are complex and unpredictable, often behaving differently outside controlled laboratory conditions. Safety concerns, regulatory frameworks, and public acceptance all require careful consideration as synthetic biology matures.pmc.ncbi.nlm.nih
Bioethics in the Genetic Age
The power to read, edit, and engineer genetic systems raises profound ethical questions. As genetic technologies become more powerful and accessible, society must grapple with fundamental questions about human enhancement, genetic equity, and the boundaries of acceptable intervention.numberanalytics+2
Germline editing—modifying genes in eggs, sperm, or early embryos—remains particularly controversial. Changes made at this level would be passed to future generations, potentially affecting the entire human species. The 2018 announcement of the first CRISPR-edited babies by Chinese researcher He Jiankui sparked international condemnation and calls for enhanced oversight.pmc.ncbi.nlm.nih+1
Genetic inequality poses another significant concern. If genetic enhancements become available but remain expensive, they could exacerbate existing social inequalities. Access to genetic therapies must be considered alongside their development to ensure broad societal benefit.numberanalytics
Privacy and discrimination concerns loom large as genetic information becomes more prevalent. While genetic anti-discrimination laws exist in many countries, enforcement and coverage gaps remain. The balance between research benefits and individual privacy continues to evolve as genetic databases grow.pmc.ncbi.nlm.nih
The Future of Genetics
As we look toward the future, several trends promise to accelerate genetic medicine’s impact. Multi-omics approaches integrate genomic data with information about RNA expression, protein levels, and metabolite concentrations, providing more complete pictures of biological systems. Artificial intelligence helps identify patterns in vast genetic datasets that would be impossible for humans to discern.wellcome+1
Gene drives—genetic modifications that spread through populations—offer potential solutions to global challenges like malaria and invasive species, but require careful consideration of ecological and ethical implications. Longevity research explores genetic factors that influence aging, potentially extending healthy human lifespan.mdpi+1
The democratization of genetic technologies continues to accelerate. Direct-to-consumer genetic testing has made genetic information accessible to millions, though interpretation and clinical utility remain challenging. Gene editing tools are becoming simpler and more affordable, potentially enabling genetic modifications outside traditional research institutions.wikipedia+2
Conclusion: The Genetic Century
From Watson and Crick’s double helix to CRISPR’s molecular scissors to personalized medicine’s tailored treatments, the past century has witnessed an extraordinary acceleration in our understanding and manipulation of life’s fundamental code. What began as a curiosity about inheritance has evolved into a comprehensive toolkit for addressing humanity’s greatest challenges.
The genetic revolution is still in its early stages. As sequencing costs continue to plummet and editing tools become more precise, genetic technologies will likely become as routine as modern surgery or pharmaceuticals. The integration of genomics with artificial intelligence, nanotechnology, and other emerging fields promises capabilities we can barely imagine today.
Yet with great power comes great responsibility. The ability to edit the code of life requires wisdom to match our technical capabilities. Ensuring equitable access to genetic therapies, protecting genetic privacy, and making thoughtful decisions about human enhancement will require ongoing dialogue between scientists, ethicists, policymakers, and the public.
The blueprint of life continues to reveal its secrets, offering unprecedented opportunities to alleviate suffering, enhance human capabilities, and understand our place in the living world. As we write the next chapters of the genetic story, we hold in our hands not just the power to read life’s instruction manual, but to rewrite it entirely. The choices we make today will determine whether genetics becomes humanity’s greatest tool for flourishing or its most dangerous burden.
In decoding DNA, we have unlocked not just the secrets of heredity, but a new chapter in human evolution—one where we become active participants in shaping our own biological future. The age of genomics has begun, and its implications will reverberate through every aspect of human society for generations to come.
Sources:-
- https://pmc.ncbi.nlm.nih.gov/articles/PMC1201016/
- https://www.genome.gov/25520255/online-education-kit-1953-dna-double-helix
- https://www.yourgenome.org/theme/the-discovery-of-dna-unravelling-the-double-helix/
- https://crisprtx.com/gene-editing
- https://en.wikipedia.org/wiki/CRISPR_gene_editing
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3281749/
- https://www.kci.go.kr/kciportal/landing/article.kci?arti_id=ART002412159
- https://journals.iucr.org/paper?S2059798322010658
- https://profiles.nlm.nih.gov/spotlight/sc/feature/doublehelix
- http://www.scholink.org/ojs/index.php/fet/article/view/22254
- https://www.nature.com/articles/d41586-023-01313-5
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3663117/
- https://chem.libretexts.org/Courses/Eastern_Mennonite_University/EMU:_Chemistry_for_the_Life_Sciences_(Cessna)/19:_Nucleic_Acids/19.4:_Protein_Synthesis_and_the_Genetic_Code
- https://en.wikipedia.org/wiki/Genetic_code
- https://byjus.com/biology/genetic-code/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3829400/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6875757/
- https://en.wikipedia.org/wiki/Human_Genome_Project
- https://www.genome.gov/about-genomics/educational-resources/fact-sheets/human-genome-project
- https://wellcome.org/news/human-genome-project-new-era-scientific-progress
- https://www.icr.ac.uk/about-us/icr-news/detail/how-the-human-genome-project-shook-the-world-of-cancer-research
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10916045/
- https://medlineplus.gov/genetics/understanding/genomicresearch/genomeediting/
- https://news.stanford.edu/stories/2024/06/stanford-explainer-crispr-gene-editing-and-beyond
- https://www.nature.com/articles/s41392-023-01440-5
- https://sapac.illumina.com/techniques/popular-applications/synthetic-biology.html
- https://www.genomicsengland.co.uk/blog/genomics-101-what-is-personalised-medicine
- https://en.wikipedia.org/wiki/Personalized_genomics
- https://www.genome.gov/genetics-glossary/Personalized-Medicine
- https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-021-01103-8
- http://www.dl.begellhouse.com/journals/439f422d0783386a,1dc3cf1839473109,6a9a4a124f9d93ab.html
- https://pmc.ncbi.nlm.nih.gov/articles/PMC5839622/
- https://medlineplus.gov/genetics/understanding/howgeneswork/epigenome/
- https://www.nature.com/articles/s41392-023-01333-7
- https://www.frontiersin.org/articles/10.3389/fcell.2020.619301/full
- https://www.nature.com/articles/hdy201054
- https://imrpress.com/journal/FBL/22/7/10.2741/4535
- https://www.mdpi.com/2072-6694/13/6/1213
- https://lifesciences.danaher.com/us/en/library/synthetic-biology.html
- https://en.wikipedia.org/wiki/Synthetic_biology
- https://pmc.ncbi.nlm.nih.gov/articles/PMC7925609/
- https://www.numberanalytics.com/blog/bioethics-of-genetic-engineering
- https://pmc.ncbi.nlm.nih.gov/articles/PMC8774098/
- https://news.harvard.edu/gazette/story/2019/01/perspectives-on-gene-editing/
- https://cssh.northeastern.edu/philosophy/wp-content/uploads/sites/11/2021/01/Sandler_Genetic-Engineering.pdf
- https://www.semanticscholar.org/paper/4bcaccb8b7853024b62e2fec6360f40abce71fee
- https://link.springer.com/10.1134/S1062360422010064
- https://intellectdiscover.com/content/journals/10.1386/pop.4.2.163_1
- https://pubs.aip.org/physicstoday/article/21/8/71/426471/The-Double-Helix-A-Personal-Account-of-the
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11112577/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6689071/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC2684691/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3464188/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6045379/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6378961/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6318791/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC4824394/
- http://ukrbiochemjournal.org/wp-content/uploads/2020/09/Danylova_TV_4_20.pdf
- https://pmc.ncbi.nlm.nih.gov/articles/PMC8063756/
- https://www.biologyonline.com/tutorials/genetic-information-and-protein-synthesis
- https://www.khanacademy.org/science/biology/dna-as-the-genetic-material/dna-discovery-and-structure/a/discovery-of-the-structure-of-dna
- https://www.youtube.com/watch?v=1vm3od_UmFg
- https://www.britannica.com/event/Human-Genome-Project
- https://www.semanticscholar.org/paper/11788d20d4bf383fb530142281cf4ab90a727cb0
- https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-018-0463-6
- http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1381-6128&volume=20&issue=11&spage=1726
- https://epigeneticsandchromatin.biomedcentral.com/articles/10.1186/s13072-018-0205-1
- http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-879X2005001000010&lng=en&tlng=en
- https://pmc.ncbi.nlm.nih.gov/articles/PMC2732403/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3257095/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC4222334/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC5293546/
- https://www.frontiersin.org/articles/10.3389/fgene.2020.00869/pdf
- https://pmc.ncbi.nlm.nih.gov/articles/PMC4222336/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC4600412/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC4323865/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC4888816/
- https://www.mdpi.com/1422-0067/12/12/8661/pdf
- https://pubmed.ncbi.nlm.nih.gov/11885124/
- https://www.genomics-aotearoa.org.nz/education-events/precision-medicine